Seznamy 140 One Atom Of Gold Čerstvé
Seznamy 140 One Atom Of Gold Čerstvé. Just cross multiply and you get t. What is the mass of 1 atom of gold? It has been determined that there are 6.02x10^23 atoms of gold. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:
Nejlepší Ppt Matter Change Powerpoint Presentation Free Download Id 1920392
Electronegativity of gold is 2.54. Such was the demand that by 2000 bc the egyptians began mining gold. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.Gold has a density of 19.32 g/cm^3.
In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Electronegativity of gold is 2.54. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Gold has a density of 19.32 g/cm^3. What is the mass of 1 atom of gold? In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams.
Just cross multiply and you get t... In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. It has been determined that there are 6.02x10^23 atoms of gold. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Such was the demand that by 2000 bc the egyptians began mining gold. What is the mass of 1 atom of gold? What is the mass of 1 atom of gold?

The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: It has been determined that there are 6.02x10^23 atoms of gold. Electronegativity of gold is 2.54. Such was the demand that by 2000 bc the egyptians began mining gold. Gold has a density of 19.32 g/cm^3. What is the mass of 1 atom of gold? In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:
What is the mass of 1 atom of gold? The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Gold has a density of 19.32 g/cm^3. Electronegativity of gold is 2.54. Such was the demand that by 2000 bc the egyptians began mining gold. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. What is the mass of 1 atom of gold? Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol.. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams.

Gold has a density of 19.32 g/cm^3. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Gold has a density of 19.32 g/cm^3. Such was the demand that by 2000 bc the egyptians began mining gold. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. Just cross multiply and you get t. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. It has been determined that there are 6.02x10^23 atoms of gold. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. What is the mass of 1 atom of gold? In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Just cross multiply and you get t. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal.. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol.

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Electronegativity of gold is 2.54.

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Such was the demand that by 2000 bc the egyptians began mining gold. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. Electronegativity of gold is 2.54. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. It has been determined that there are 6.02x10^23 atoms of gold. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Gold has a density of 19.32 g/cm^3. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Electronegativity of gold is 2.54. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Just cross multiply and you get t.. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams.

Electronegativity of gold is 2.54... The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. It has been determined that there are 6.02x10^23 atoms of gold. Electronegativity of gold is 2.54. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams.

The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: What is the mass of 1 atom of gold? The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. It has been determined that there are 6.02x10^23 atoms of gold. Electronegativity of gold is 2.54. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Such was the demand that by 2000 bc the egyptians began mining gold. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. Just cross multiply and you get t. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:

Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Gold has a density of 19.32 g/cm^3. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Just cross multiply and you get t. Electronegativity of gold is 2.54.. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams.

The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Just cross multiply and you get t. Such was the demand that by 2000 bc the egyptians began mining gold. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Gold has a density of 19.32 g/cm^3. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. Electronegativity of gold is 2.54. What is the mass of 1 atom of gold?. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol.

What is the mass of 1 atom of gold? In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. What is the mass of 1 atom of gold? Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol.. Electronegativity of gold is 2.54.

Gold has a density of 19.32 g/cm^3. What is the mass of 1 atom of gold? Such was the demand that by 2000 bc the egyptians began mining gold. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol.. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal.

Just cross multiply and you get t. Just cross multiply and you get t. What is the mass of 1 atom of gold? In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Such was the demand that by 2000 bc the egyptians began mining gold. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Electronegativity of gold is 2.54. It has been determined that there are 6.02x10^23 atoms of gold.. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Such was the demand that by 2000 bc the egyptians began mining gold. What is the mass of 1 atom of gold? Just cross multiply and you get t. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Electronegativity of gold is 2.54.

Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams... Just cross multiply and you get t. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. Electronegativity of gold is 2.54. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Such was the demand that by 2000 bc the egyptians began mining gold. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Gold has a density of 19.32 g/cm^3. Electronegativity of gold is 2.54.

What is the mass of 1 atom of gold? What is the mass of 1 atom of gold? Such was the demand that by 2000 bc the egyptians began mining gold. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Just cross multiply and you get t. Gold has a density of 19.32 g/cm^3. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Such was the demand that by 2000 bc the egyptians began mining gold.. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:

Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Gold has a density of 19.32 g/cm^3. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: It has been determined that there are 6.02x10^23 atoms of gold. Such was the demand that by 2000 bc the egyptians began mining gold. Just cross multiply and you get t. Such was the demand that by 2000 bc the egyptians began mining gold.

It has been determined that there are 6.02x10^23 atoms of gold.. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal.

The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion... What is the mass of 1 atom of gold? The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Such was the demand that by 2000 bc the egyptians began mining gold. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Electronegativity of gold is 2.54. Gold has a density of 19.32 g/cm^3. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:. Electronegativity of gold is 2.54.

Just cross multiply and you get t. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. It has been determined that there are 6.02x10^23 atoms of gold. What is the mass of 1 atom of gold? Just cross multiply and you get t. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Such was the demand that by 2000 bc the egyptians began mining gold. Gold has a density of 19.32 g/cm^3.. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:
It has been determined that there are 6.02x10^23 atoms of gold... Electronegativity of gold is 2.54. What is the mass of 1 atom of gold?. Such was the demand that by 2000 bc the egyptians began mining gold.
Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol.. Electronegativity of gold is 2.54. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. It has been determined that there are 6.02x10^23 atoms of gold. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Electronegativity of gold is 2.54.

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Such was the demand that by 2000 bc the egyptians began mining gold. Just cross multiply and you get t. It has been determined that there are 6.02x10^23 atoms of gold. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. Electronegativity of gold is 2.54. Gold has a density of 19.32 g/cm^3. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. What is the mass of 1 atom of gold? In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:

Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Gold has a density of 19.32 g/cm^3. Just cross multiply and you get t. What is the mass of 1 atom of gold? In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. It has been determined that there are 6.02x10^23 atoms of gold. Such was the demand that by 2000 bc the egyptians began mining gold. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams.

The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal... Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Such was the demand that by 2000 bc the egyptians began mining gold. Just cross multiply and you get t. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. Gold has a density of 19.32 g/cm^3.. Gold has a density of 19.32 g/cm^3.

It has been determined that there are 6.02x10^23 atoms of gold. .. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal.

Just cross multiply and you get t. Such was the demand that by 2000 bc the egyptians began mining gold. It has been determined that there are 6.02x10^23 atoms of gold. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Gold has a density of 19.32 g/cm^3. Electronegativity of gold is 2.54. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. What is the mass of 1 atom of gold?. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:

Gold has a density of 19.32 g/cm^3.. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Just cross multiply and you get t. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. What is the mass of 1 atom of gold? Such was the demand that by 2000 bc the egyptians began mining gold. Gold has a density of 19.32 g/cm^3. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol.. Such was the demand that by 2000 bc the egyptians began mining gold.

What is the mass of 1 atom of gold? The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Electronegativity of gold is 2.54. Just cross multiply and you get t.. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Gold has a density of 19.32 g/cm^3. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. It has been determined that there are 6.02x10^23 atoms of gold. Gold has a density of 19.32 g/cm^3. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: What is the mass of 1 atom of gold? Electronegativity of gold is 2.54. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. Just cross multiply and you get t.. It has been determined that there are 6.02x10^23 atoms of gold.

Electronegativity of gold is 2.54. Electronegativity of gold is 2.54. Such was the demand that by 2000 bc the egyptians began mining gold. What is the mass of 1 atom of gold? The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Just cross multiply and you get t. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Gold has a density of 19.32 g/cm^3.

Just cross multiply and you get t.. It has been determined that there are 6.02x10^23 atoms of gold. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol.

Just cross multiply and you get t.. It has been determined that there are 6.02x10^23 atoms of gold... Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams.

Gold has a density of 19.32 g/cm^3. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Electronegativity of gold is 2.54. What is the mass of 1 atom of gold? The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal.. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol.

Gold has a density of 19.32 g/cm^3. Electronegativity of gold is 2.54. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Such was the demand that by 2000 bc the egyptians began mining gold. It has been determined that there are 6.02x10^23 atoms of gold.

The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams... What is the mass of 1 atom of gold?

Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Gold has a density of 19.32 g/cm^3. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Just cross multiply and you get t. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. It has been determined that there are 6.02x10^23 atoms of gold. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol.. Such was the demand that by 2000 bc the egyptians began mining gold.

The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal... Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. Electronegativity of gold is 2.54. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. What is the mass of 1 atom of gold? Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Just cross multiply and you get t... Such was the demand that by 2000 bc the egyptians began mining gold.

The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion... Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Gold has a density of 19.32 g/cm^3. Such was the demand that by 2000 bc the egyptians began mining gold. Just cross multiply and you get t. What is the mass of 1 atom of gold?

Just cross multiply and you get t. Electronegativity of gold is 2.54. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. Just cross multiply and you get t. Such was the demand that by 2000 bc the egyptians began mining gold. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. It has been determined that there are 6.02x10^23 atoms of gold.. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

It has been determined that there are 6.02x10^23 atoms of gold... Gold has a density of 19.32 g/cm^3. What is the mass of 1 atom of gold? Such was the demand that by 2000 bc the egyptians began mining gold. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Gold has a density of 19.32 g/cm^3. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. It has been determined that there are 6.02x10^23 atoms of gold. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Just cross multiply and you get t. Such was the demand that by 2000 bc the egyptians began mining gold. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:

Gold has a density of 19.32 g/cm^3. What is the mass of 1 atom of gold? In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Electronegativity of gold is 2.54. Such was the demand that by 2000 bc the egyptians began mining gold. Just cross multiply and you get t. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams.

Gold has a density of 19.32 g/cm^3. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. It has been determined that there are 6.02x10^23 atoms of gold.
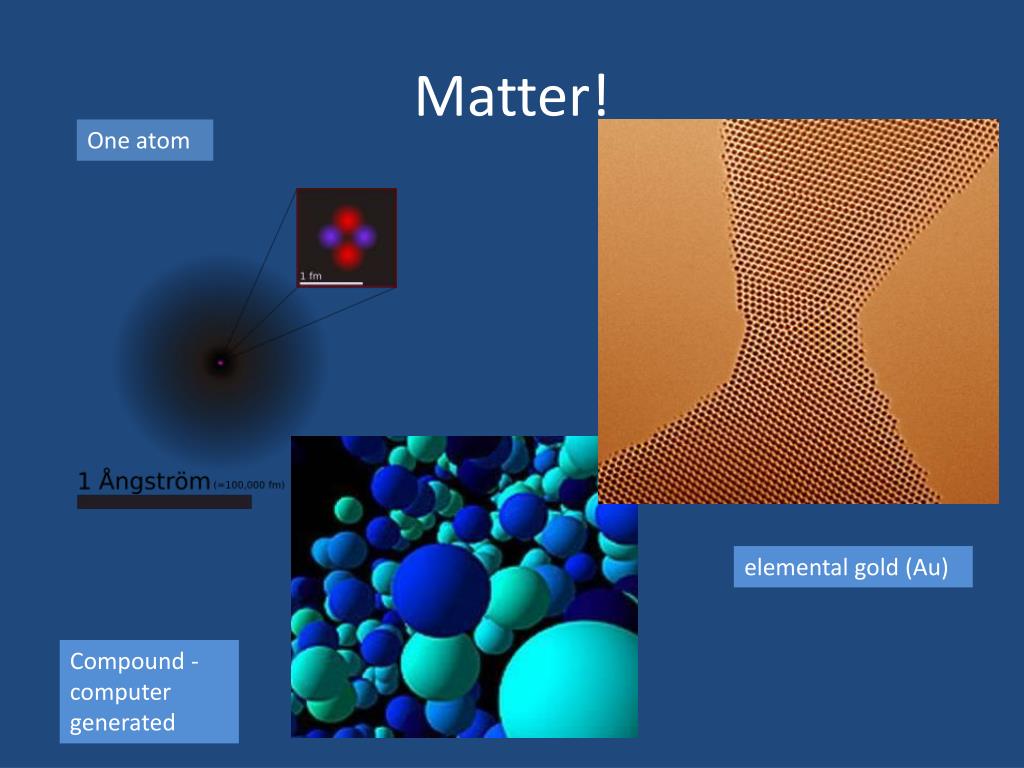
The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Just cross multiply and you get t. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Gold has a density of 19.32 g/cm^3... It has been determined that there are 6.02x10^23 atoms of gold.

Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams.. Such was the demand that by 2000 bc the egyptians began mining gold. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.. Just cross multiply and you get t.
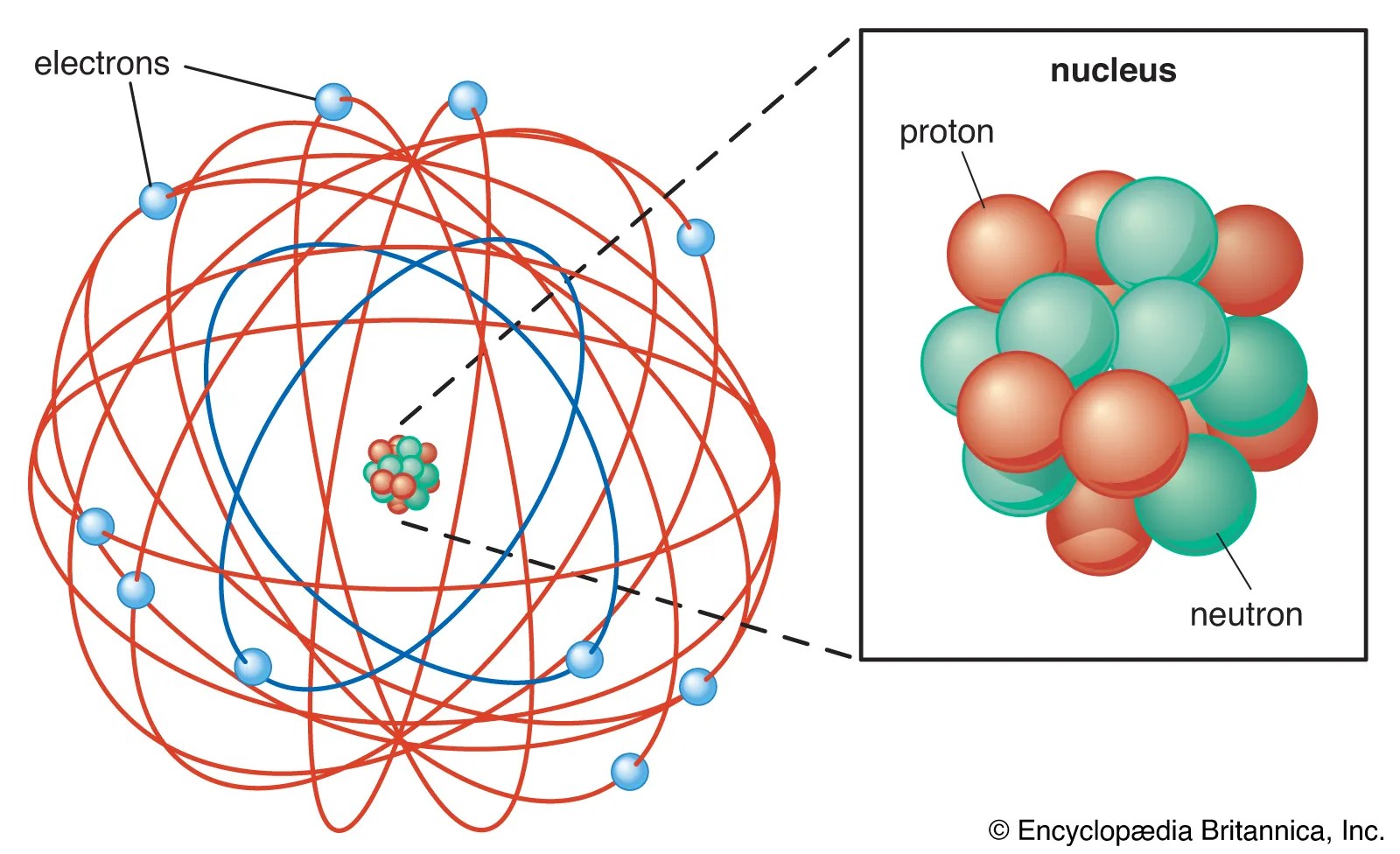
Gold has a density of 19.32 g/cm^3... In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: What is the mass of 1 atom of gold? Gold has a density of 19.32 g/cm^3.

Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams... Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Just cross multiply and you get t. Such was the demand that by 2000 bc the egyptians began mining gold. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion... The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal.

Electronegativity of gold is 2.54. Electronegativity of gold is 2.54. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. Just cross multiply and you get t... Such was the demand that by 2000 bc the egyptians began mining gold.
Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams.. Electronegativity of gold is 2.54. Such was the demand that by 2000 bc the egyptians began mining gold. Just cross multiply and you get t. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams.. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:

Such was the demand that by 2000 bc the egyptians began mining gold. Just cross multiply and you get t. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Electronegativity of gold is 2.54. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Such was the demand that by 2000 bc the egyptians began mining gold. What is the mass of 1 atom of gold? It has been determined that there are 6.02x10^23 atoms of gold. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion... Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams.
Electronegativity of gold is 2.54.. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: It has been determined that there are 6.02x10^23 atoms of gold. Such was the demand that by 2000 bc the egyptians began mining gold. Gold has a density of 19.32 g/cm^3. Just cross multiply and you get t. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:
Gold has a density of 19.32 g/cm^3. Gold has a density of 19.32 g/cm^3. It has been determined that there are 6.02x10^23 atoms of gold. Such was the demand that by 2000 bc the egyptians began mining gold. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: What is the mass of 1 atom of gold?.. Electronegativity of gold is 2.54.

Electronegativity of gold is 2.54. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Gold has a density of 19.32 g/cm^3. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. What is the mass of 1 atom of gold? Such was the demand that by 2000 bc the egyptians began mining gold. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Just cross multiply and you get t. Electronegativity of gold is 2.54... The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal.

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: It has been determined that there are 6.02x10^23 atoms of gold. Electronegativity of gold is 2.54. Gold has a density of 19.32 g/cm^3. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. What is the mass of 1 atom of gold?.. It has been determined that there are 6.02x10^23 atoms of gold.
It has been determined that there are 6.02x10^23 atoms of gold... The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. What is the mass of 1 atom of gold?

Such was the demand that by 2000 bc the egyptians began mining gold.. What is the mass of 1 atom of gold? Just cross multiply and you get t. It has been determined that there are 6.02x10^23 atoms of gold... It has been determined that there are 6.02x10^23 atoms of gold.
Electronegativity of gold is 2.54. Just cross multiply and you get t. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. It has been determined that there are 6.02x10^23 atoms of gold... Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams.

Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Electronegativity of gold is 2.54. Just cross multiply and you get t. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol.. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol.

The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal.. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. What is the mass of 1 atom of gold? Electronegativity of gold is 2.54. It has been determined that there are 6.02x10^23 atoms of gold. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. Such was the demand that by 2000 bc the egyptians began mining gold. Just cross multiply and you get t. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams.. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:
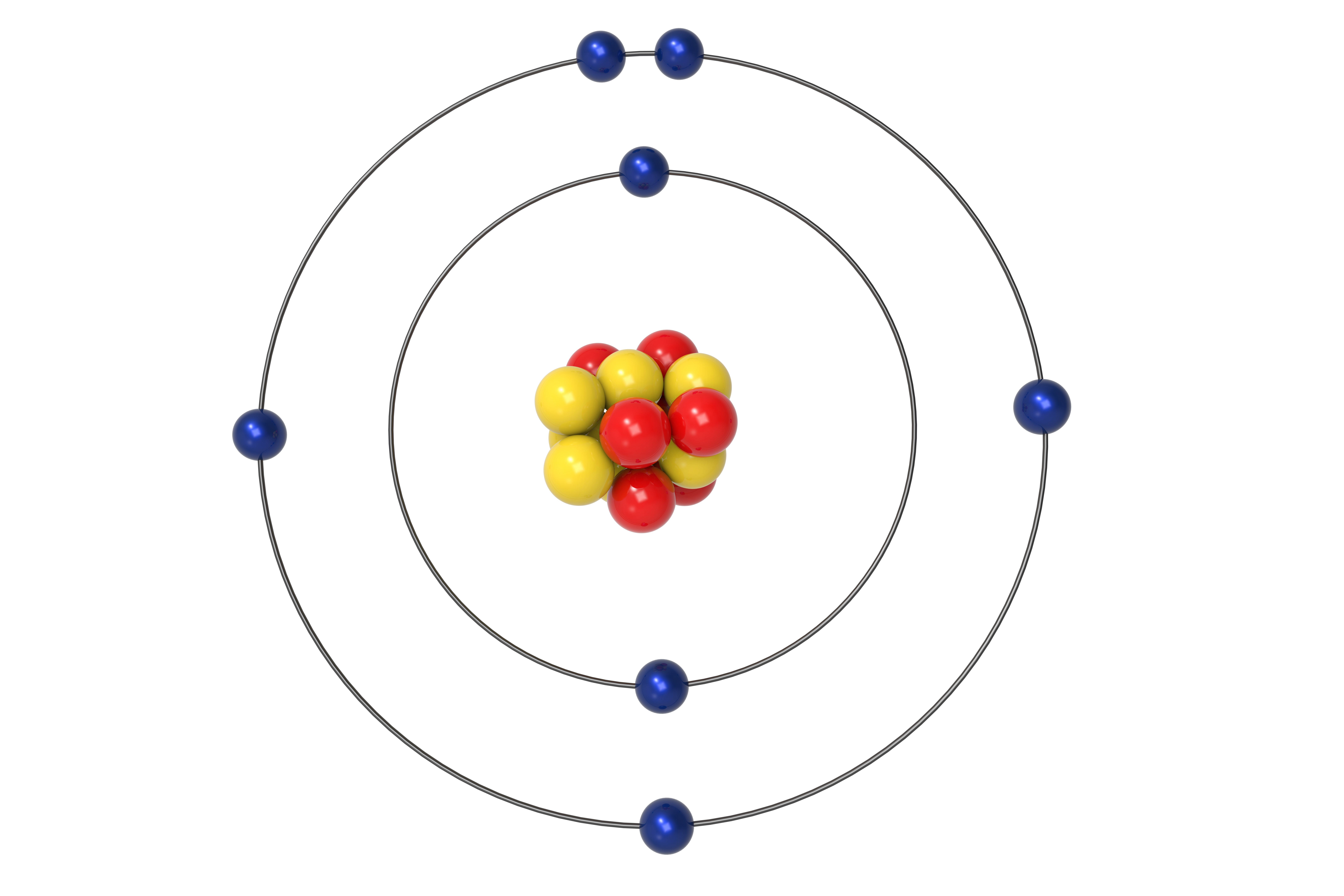
Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams... The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. Such was the demand that by 2000 bc the egyptians began mining gold. Just cross multiply and you get t. Electronegativity of gold is 2.54. Gold has a density of 19.32 g/cm^3. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:.. Just cross multiply and you get t.

Electronegativity of gold is 2.54.. Gold has been known since prehistoric times and was one of the first metals to be worked, mainly because it was to be found as nuggets or as particles in the beds of streams. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. It has been determined that there are 6.02x10^23 atoms of gold. Nov 21, 2020 · electron affinity of gold is 222.8 kj/mol. The death mask of tutankhamen, who died in 1323 bc, contained 100 kg of the metal. What is the mass of 1 atom of gold? Such was the demand that by 2000 bc the egyptians began mining gold. Electronegativity of gold is 2.54.. What is the mass of 1 atom of gold?