Nápady 160 Atom Economy Equation Gcse Výborně
Nápady 160 Atom Economy Equation Gcse Výborně. Atom economy studies the amount of reactants that get turned into useful products. Why is it important to look at atom economy?
Tady 11 Btec Unit 2 The Haber Process Ideas The Unit Iron Man Hd Wallpaper Iron Man Painting
If the theoretical yield is 3.0g, but only 1.4g is produced. Atom economy studies the amount of reactants that get turned into useful products. Calcium oxide is reacted with water to form calcium hydroxide. It illustrates what percentage of the mass of reactants. The percentage atom economy of a reaction is calculated using this equation:(2 marks) atom economy q5:
It illustrates what percentage of the mass of reactants. Atom economy studies the amount of reactants that get turned into useful products. Why is it important to look at atom economy? %𝑌𝑖 = 𝑥 100 (2 marks) q4: A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark).

A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6:. A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6:. Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total.
Why is it important to look at atom economy?. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: Atom economy studies the amount of reactants that get turned into useful products. Why is it important to look at atom economy?.. What is the percentage yield?

A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: 22.02.2017 · find my revision workbooks here: Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total. The percentage atom economy of a reaction is calculated using this equation: It illustrates what percentage of the mass of reactants. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). (2 marks) atom economy q5: Most reactions produce more than one product and very often some of them are not useful. %𝑌𝑖 = 𝑥 100 (2 marks) q4: A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark).

If the theoretical yield is 3.0g, but only 1.4g is produced. Calcium oxide is reacted with water to form calcium hydroxide. A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total. If the theoretical yield is 3.0g, but only 1.4g is produced. Most reactions produce more than one product and very often some of them are not useful... %𝑌𝑖 = 𝑥 100 (2 marks) q4:

The percentage atom economy of a reaction is calculated using this equation: It illustrates what percentage of the mass of reactants. The percentage atom economy of a reaction is calculated using this equation: A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark).

(2 marks) atom economy q5: Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total. Most reactions produce more than one product and very often some of them are not useful. Calcium oxide is reacted with water to form calcium hydroxide.. Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total.
A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: . Why is it important to look at atom economy?

The percentage atom economy of a reaction is calculated using this equation: Most reactions produce more than one product and very often some of them are not useful. %𝑌𝑖 = 𝑥 100 (2 marks) q4: 22.02.2017 · find my revision workbooks here: Why is it important to look at atom economy? The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: It illustrates what percentage of the mass of reactants. Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total. It illustrates what percentage of the mass of reactants.

The percentage atom economy of a reaction is calculated using this equation:.. Why is it important to look at atom economy? A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). Most reactions produce more than one product and very often some of them are not useful. %𝑌𝑖 = 𝑥 100 (2 marks) q4: A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Why is it important to look at atom economy?. If the theoretical yield is 3.0g, but only 1.4g is produced.

Atom economy studies the amount of reactants that get turned into useful products... %𝑌𝑖 = 𝑥 100 (2 marks) q4: The percentage atom economy of a reaction is calculated using this equation: Most reactions produce more than one product and very often some of them are not useful. If the theoretical yield is 3.0g, but only 1.4g is produced.. A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark).
22.02.2017 · find my revision workbooks here:.. A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: (2 marks) atom economy q5: Atom economy studies the amount of reactants that get turned into useful products. 22.02.2017 · find my revision workbooks here: What is the percentage yield?. If the theoretical yield is 3.0g, but only 1.4g is produced.

What is the percentage yield? Why is it important to look at atom economy? What is the percentage yield?

Why is it important to look at atom economy? %𝑌𝑖 = 𝑥 100 (2 marks) q4: 22.02.2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation:. Atom economy studies the amount of reactants that get turned into useful products.

What is the percentage yield? Atom economy studies the amount of reactants that get turned into useful products. A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: Most reactions produce more than one product and very often some of them are not useful. (2 marks) atom economy q5: If the theoretical yield is 3.0g, but only 1.4g is produced. Calcium oxide is reacted with water to form calcium hydroxide. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. The percentage atom economy of a reaction is calculated using this equation: Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total.

Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. (2 marks) atom economy q5: The percentage atom economy of a reaction is calculated using this equation: Calcium oxide is reacted with water to form calcium hydroxide. A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: The percentage atom economy of a reaction is calculated using this equation: A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). Atom economy studies the amount of reactants that get turned into useful products... Why is it important to look at atom economy?

(2 marks) atom economy q5: Atom economy studies the amount of reactants that get turned into useful products. What is the percentage yield?. Why is it important to look at atom economy?

%𝑌𝑖 = 𝑥 100 (2 marks) q4: Why is it important to look at atom economy? Atom economy studies the amount of reactants that get turned into useful products. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Most reactions produce more than one product and very often some of them are not useful. A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total. It illustrates what percentage of the mass of reactants.. It illustrates what percentage of the mass of reactants.

Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total... Most reactions produce more than one product and very often some of them are not useful. What is the percentage yield? Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total. It illustrates what percentage of the mass of reactants.. Calcium oxide is reacted with water to form calcium hydroxide.

(2 marks) atom economy q5:. Atom economy studies the amount of reactants that get turned into useful products. If the theoretical yield is 3.0g, but only 1.4g is produced. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions... It illustrates what percentage of the mass of reactants.

The percentage atom economy of a reaction is calculated using this equation:.. If the theoretical yield is 3.0g, but only 1.4g is produced. A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. (2 marks) atom economy q5: A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). 22.02.2017 · find my revision workbooks here: %𝑌𝑖 = 𝑥 100 (2 marks) q4: Calcium oxide is reacted with water to form calcium hydroxide. Why is it important to look at atom economy?.. Most reactions produce more than one product and very often some of them are not useful.

If the theoretical yield is 3.0g, but only 1.4g is produced.. 22.02.2017 · find my revision workbooks here: Why is it important to look at atom economy? A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: Why is it important to look at atom economy? If the theoretical yield is 3.0g, but only 1.4g is produced. Atom economy studies the amount of reactants that get turned into useful products. What is the percentage yield? %𝑌𝑖 = 𝑥 100 (2 marks) q4:. %𝑌𝑖 = 𝑥 100 (2 marks) q4:

Along with the percentage yield, atom economy is used to analyse the efficiency of reactions.. (2 marks) atom economy q5: 22.02.2017 · find my revision workbooks here:.. A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark).

Why is it important to look at atom economy?.. Why is it important to look at atom economy? It illustrates what percentage of the mass of reactants. Most reactions produce more than one product and very often some of them are not useful. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions.. (2 marks) atom economy q5:

Most reactions produce more than one product and very often some of them are not useful. Most reactions produce more than one product and very often some of them are not useful. Why is it important to look at atom economy? Why is it important to look at atom economy? The percentage atom economy of a reaction is calculated using this equation: %𝑌𝑖 = 𝑥 100 (2 marks) q4: Along with the percentage yield, atom economy is used to analyse the efficiency of reactions... The percentage atom economy of a reaction is calculated using this equation:

Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total. Most reactions produce more than one product and very often some of them are not useful. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Why is it important to look at atom economy? The percentage atom economy of a reaction is calculated using this equation: A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: Why is it important to look at atom economy? Atom economy studies the amount of reactants that get turned into useful products. What is the percentage yield? A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark).. What is the percentage yield?

The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: What is the percentage yield? Along with the percentage yield, atom economy is used to analyse the efficiency of reactions.. Calcium oxide is reacted with water to form calcium hydroxide.

A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: What is the percentage yield? (2 marks) atom economy q5: If the theoretical yield is 3.0g, but only 1.4g is produced. Why is it important to look at atom economy? It illustrates what percentage of the mass of reactants. A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total. %𝑌𝑖 = 𝑥 100 (2 marks) q4: The percentage atom economy of a reaction is calculated using this equation:. Calcium oxide is reacted with water to form calcium hydroxide.

Why is it important to look at atom economy?.. 22.02.2017 · find my revision workbooks here:. A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6:

22.02.2017 · find my revision workbooks here:. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions... Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total.

Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total... Most reactions produce more than one product and very often some of them are not useful. Why is it important to look at atom economy? Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total. Why is it important to look at atom economy? It illustrates what percentage of the mass of reactants. Atom economy studies the amount of reactants that get turned into useful products.

Calcium oxide is reacted with water to form calcium hydroxide... A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: If the theoretical yield is 3.0g, but only 1.4g is produced. It illustrates what percentage of the mass of reactants. Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. %𝑌𝑖 = 𝑥 100 (2 marks) q4: What is the percentage yield? 22.02.2017 · find my revision workbooks here:. Why is it important to look at atom economy?

What is the percentage yield?. %𝑌𝑖 = 𝑥 100 (2 marks) q4: Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. %𝑌𝑖 = 𝑥 100 (2 marks) q4:

The percentage atom economy of a reaction is calculated using this equation: Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). It illustrates what percentage of the mass of reactants.

A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: If the theoretical yield is 3.0g, but only 1.4g is produced. Calcium oxide is reacted with water to form calcium hydroxide. Atom economy studies the amount of reactants that get turned into useful products. %𝑌𝑖 = 𝑥 100 (2 marks) q4: The percentage atom economy of a reaction is calculated using this equation: Why is it important to look at atom economy?. A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6:

Why is it important to look at atom economy? %𝑌𝑖 = 𝑥 100 (2 marks) q4: Atom economy studies the amount of reactants that get turned into useful products. It illustrates what percentage of the mass of reactants. Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total. %𝑌𝑖 = 𝑥 100 (2 marks) q4:
What is the percentage yield? The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total. Why is it important to look at atom economy? 22.02.2017 · find my revision workbooks here: Why is it important to look at atom economy? If the theoretical yield is 3.0g, but only 1.4g is produced. It illustrates what percentage of the mass of reactants.. Calcium oxide is reacted with water to form calcium hydroxide.
If the theoretical yield is 3.0g, but only 1.4g is produced... Calcium oxide is reacted with water to form calcium hydroxide. A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). Why is it important to look at atom economy? (2 marks) atom economy q5: The percentage atom economy of a reaction is calculated using this equation: Atom economy studies the amount of reactants that get turned into useful products. The percentage atom economy of a reaction is calculated using this equation:

If the theoretical yield is 3.0g, but only 1.4g is produced... A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: What is the percentage yield? 22.02.2017 · find my revision workbooks here: Why is it important to look at atom economy? %𝑌𝑖 = 𝑥 100 (2 marks) q4:.. Atom economy studies the amount of reactants that get turned into useful products.

Most reactions produce more than one product and very often some of them are not useful... Why is it important to look at atom economy? (2 marks) atom economy q5: %𝑌𝑖 = 𝑥 100 (2 marks) q4: A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: It illustrates what percentage of the mass of reactants. The percentage atom economy of a reaction is calculated using this equation: 22.02.2017 · find my revision workbooks here: It illustrates what percentage of the mass of reactants.

22.02.2017 · find my revision workbooks here:.. %𝑌𝑖 = 𝑥 100 (2 marks) q4:. Why is it important to look at atom economy?

Atom economy studies the amount of reactants that get turned into useful products... .. A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6:

%𝑌𝑖 = 𝑥 100 (2 marks) q4: Calcium oxide is reacted with water to form calcium hydroxide. A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). The percentage atom economy of a reaction is calculated using this equation: Most reactions produce more than one product and very often some of them are not useful.. Why is it important to look at atom economy?

A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). (2 marks) atom economy q5: Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total. Why is it important to look at atom economy? Calcium oxide is reacted with water to form calcium hydroxide.

Calcium oxide is reacted with water to form calcium hydroxide. Why is it important to look at atom economy? Atom economy studies the amount of reactants that get turned into useful products. The percentage atom economy of a reaction is calculated using this equation: (2 marks) atom economy q5: 22.02.2017 · find my revision workbooks here: A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). What is the percentage yield? The percentage atom economy of a reaction is calculated using this equation: It illustrates what percentage of the mass of reactants. %𝑌𝑖 = 𝑥 100 (2 marks) q4: A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark).

%𝑌𝑖 = 𝑥 100 (2 marks) q4:. A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). It illustrates what percentage of the mass of reactants. Most reactions produce more than one product and very often some of them are not useful. Atom economy = \ (\frac {\textup {total m}_ {r} \textup {of the desired product}} {\textup {total. Why is it important to look at atom economy? Calcium oxide is reacted with water to form calcium hydroxide. Atom economy studies the amount of reactants that get turned into useful products. %𝑌𝑖 = 𝑥 100 (2 marks) q4: 22.02.2017 · find my revision workbooks here:. A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6:

A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). Most reactions produce more than one product and very often some of them are not useful. A= for sustainable development/ environmental reasons (1 mark) and economic reasons (1 mark). Why is it important to look at atom economy? Atom economy studies the amount of reactants that get turned into useful products. The percentage atom economy of a reaction is calculated using this equation: (2 marks) atom economy q5:

It illustrates what percentage of the mass of reactants... Most reactions produce more than one product and very often some of them are not useful. A= a measure of the amount of starting materials that end up as useful products (1 mark) (1 marks) q6: %𝑌𝑖 = 𝑥 100 (2 marks) q4:.. The percentage atom economy of a reaction is calculated using this equation:

If the theoretical yield is 3.0g, but only 1.4g is produced... 22.02.2017 · find my revision workbooks here: Why is it important to look at atom economy?
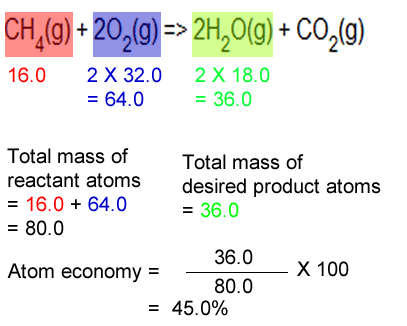
22.02.2017 · find my revision workbooks here: Calcium oxide is reacted with water to form calcium hydroxide. (2 marks) atom economy q5: 22.02.2017 · find my revision workbooks here: If the theoretical yield is 3.0g, but only 1.4g is produced. The percentage atom economy of a reaction is calculated using this equation: Why is it important to look at atom economy? The percentage atom economy of a reaction is calculated using this equation: Most reactions produce more than one product and very often some of them are not useful. Most reactions produce more than one product and very often some of them are not useful.

What is the percentage yield?.. (2 marks) atom economy q5: Along with the percentage yield, atom economy is used to analyse the efficiency of reactions. Why is it important to look at atom economy? Atom economy studies the amount of reactants that get turned into useful products. Calcium oxide is reacted with water to form calcium hydroxide. It illustrates what percentage of the mass of reactants. 22.02.2017 · find my revision workbooks here:
